Geography Specific Regulations. How to control documents more effectively? What is the purpose of SOP?
In the absence of clearly established policies and procedures for document control , several problems can arise ranging from mere confusion to grave financial losses. The document control representative shall maintain a master log of project or. The Manual provides details on the procedures to be followed and the documents to be used for each NFIP map action.

Implement and Establish the process. However, a written procedure detailing your approach to document control is not enough. This procedure related to all documents associated with providing evidence of conformity to requirements.
Records are a special type of document and shall be controlled according to the procedure for Control of Records TK-QP-102. Much more than documents. Discover everything Scribd has to offer, including books and audiobooks from major publishers. Start Free Trial Cancel anytime.
DOCUMENT CONTROL PROCEDURE REV: E. This can include processes, policy, metadata and toolsets such as document management systems designed to make documents secure, available and useful.
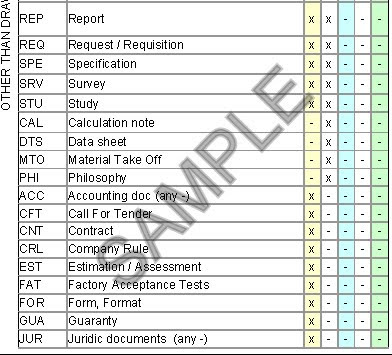
COMPANY PROPRIETARY INFORMATION This document is an uncontrolled copy. Records management is the process for providing evidence of those activities. These documents have been developed specifically for our institutions and may not be appropriate for implementation in other settings. This information is made available for professional education purposes only and each piece of information should be carefully evaluated before being adapted to meet the needs of other facilities or settings. It is a road map to track, ad archive, and remove the documents from the system.
Chemical Speciation Program 1. Document Control Procedure Accounting. Purpose and Applicability. The web version of all QMS documents and key business processes is the latest version. The purpose of this procedure is also provide guidance on the numbering of all technical and management documents generated in this Project to ensure each document has a unique reference and may be prepare tracked and managed effectively. Examples: Equipment manuals regarding the operation, safety, repair or maintenance of equipment used by QNP in the processing of customer orders.
Medical device companies are free to structure the process however they choose, but the end result must be compliant with legal requirements or relevant standards. N format, where M is the major version number to represent the major. As such, learning how to create a document management system is critical for businesses. The author of the document will ensure the date the document is created or revised is identified on the first page an when possible, is incorporated into the header or footer of the document and appears on every succeeding page.
This way you are always assured of documentation that correctly reflects the procedures. Thus, document control SOP software is all about doing business the right way. As the saying goes: “If it’s not documente it didn’t happen.
The DCC will ensure that the all procedures on the SESD LAN and the public access location of the SESD web site are updated.

The Relationship Between Processes, Procedures and Work Instructions.
No comments:
Post a Comment
Note: Only a member of this blog may post a comment.