What is the formula to calculate atomic mass? What does P on the periodic table mean? How to read the periodic table? The elemenents of the periodic table sorted by atomic mass.
The lightest chemical element is Hydrogen and the heaviest is Hassium. The unity for atomic mass is gram per mol. There you can find the metals, semi-conductor (s), non-metal (s),.
With a standard atomic weight of circa 1. Its monatomic form (H) is the most abundant chemical substance in the Universe, constituting roughly of all baryonic mass. These elements are arranged in rows and columns - left to right and top to bottom. Periodic Table with Atomic Mass. Notes on the Atomic Mass of particular elements: TechnetiuAtomic mass number given for longest lived isotope. PoloniuAtomic mass number given for longest lived isotope.

Astatine: Atomic mass number given for longest lived isotope. Radon: Atomic mass number given for longest lived isotope. The periodic table is said to be the most important part of chemistry as it makes it. All the elements are arranged in an informative order. They are positioned in the order from left to right and also top to bottom in increasing order of their atomic numbers.
This order coincides with the increase in their atomic mass. Award winning periodic table , by relative atomic mass , with user-friendly element data and facts. Each atomic mass is rounded to two decimal places for easy calculations. Interactive periodic table with dynamic layouts showing names, electrons, oxidation, trend visualization, orbitals, isotopes, and compound search.
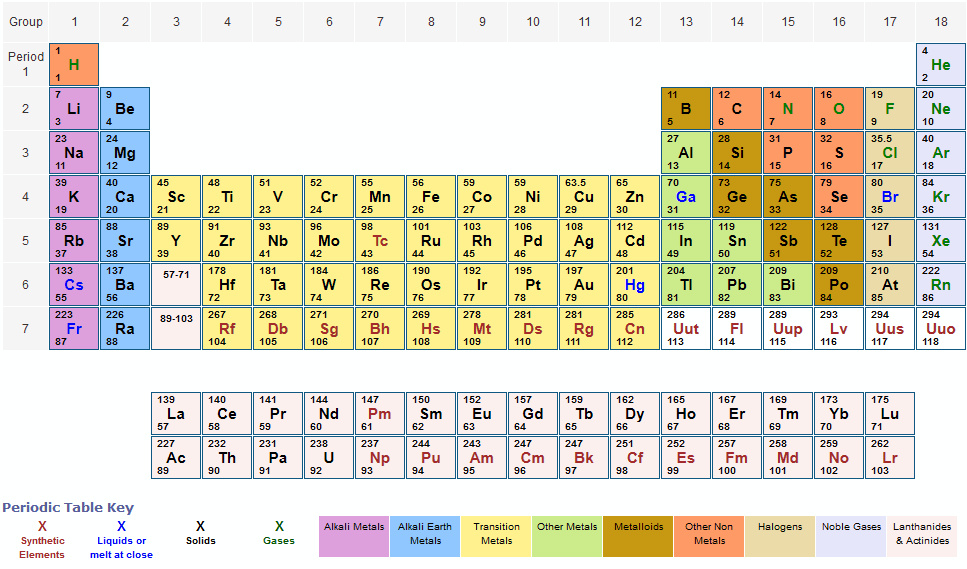
Full descriptions from write-up sources. The structure of the table shows periodic trends. Metals reside on the left side of the table , while non-metals reside on the right.
Atomic Symbol The atomic symbol is one or two letters chosen to represent an element. Standard Atomic Weight The standard atomic weight is the average mass of an element in atomic mass. The atomic weights listed on this Table of Elements have been rounded to the nearest whole number. As a result, this chart actually displays the mass number of a specific isotope for each element.
Hydrogen, at the upper left of the table , has an atomic number of 1. Every hydrogen atom has one proton in its nucleus. Atomic mass is measured in Atomic Mass Units (amu) which are scaled relative to carbon, C, that is taken as a standard element with an atomic mass of 12. This isotope of carbon has protons and neutrons.
Thus, each proton and neutron has a mass of about amu. Elements are arranged from left to right and top to bottom in order of increasing atomic number. Order generally coincides with increasing atomic mass.
The rows are called periods.
No comments:
Post a Comment
Note: Only a member of this blog may post a comment.