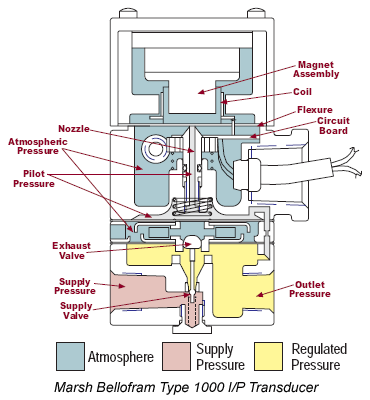
This type of corrosion inhibitor acts by forming a protective oxide film on the surface of the metal. What are reversible inhibitors? It causes a large anodic shift that forces the metallic surface into the passivation region, which reduces the corrosion potential of the material. Some examples are chromates , nitrates , molybdates , and tungstate.
Examples for Cathodic Inhibitors include sulfite and bisulfite ions which can react with oxygen to form sulphates.
Another example of a cathodic inhibitor is a catalyzed redox reaction by nickel. These types of corrosion inhibitors form a thin preventive oxide layer on the surface of the metal. Types of corrosion inhibitors: Anodic inhibitors react with oxygen to form a thin film on the surface of the metal. They reduce the corrosion potential of the material by oxidizing a surface layer that is less reactive to corrosive elements.
Cathodic inhibitors slow the cathodic reaction itself or limit the diffusion of reductive elements such as hydrogen or oxygen to the metal surface. Examples are cathodic poisons such as arsenic and selenium ions which slow the reaction or oxygen. In this example, hydrazine converts oxygen , a common corrosive agent, to water, which is generally benign.